

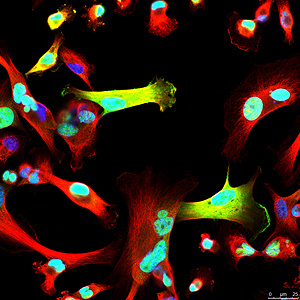
Celina Kleer, MD’s lab in the Rogel Cancer Center is occupied by staff and students with a range of experiences and talents, but a common goal – to understand what triggers breast cancer progression. A physician-scientist, Kleer specializes in breast pathology. Having witnessed first-hand that certain breast cancers have the ability to metastasize early and rapidly compared to others, has inspired her research program at U-M. Her most recent challenge, triple-negative breast cancers, are particularly aggressive with a high propensity for distant dissemination. Kleer says that the molecular basis of metastasis of triple negative breast cancer has remained elusive, explaining “It is important to understand the mechanisms that are underlying these tumors’ aggressive behavior so we can ultimately inhibit metastases.”
 Anwar TalhaAn aspiring physician, Talha Anwar, had just completed his undergrad at Brown University and was working at the National Institutes of Health when he realized he wanted research to be a part of his career as well. He was looking for a school that had strengths both in clinical medicine and basic science research. That desire led him to U-M’s MD/ PhD Medical Scientist Training Program (MSTP). This joint effort between U-M’s medical and graduate schools, allows students to graduate with both degrees after completing the curriculum over seven to eight full calendar years, including summer lab rotations.
Anwar TalhaAn aspiring physician, Talha Anwar, had just completed his undergrad at Brown University and was working at the National Institutes of Health when he realized he wanted research to be a part of his career as well. He was looking for a school that had strengths both in clinical medicine and basic science research. That desire led him to U-M’s MD/ PhD Medical Scientist Training Program (MSTP). This joint effort between U-M’s medical and graduate schools, allows students to graduate with both degrees after completing the curriculum over seven to eight full calendar years, including summer lab rotations.
Anwar first rotated through the Kleer Lab at the end of his first year of medical school in 2012 and recalls that Kleer was very influential in his decision to study cancer biology as part of the Molecular and Cellular Pathology (MCP) program for the PhD portion of his training. This proved to be a wise decision, as the MCP program offered a collegial and collaborative environment where he thrived. With his first two years of medical school complete and Kleer as his mentor in the MCP program, Anwar settled into his work in the lab focusing on the function of a protein called Enhancer of Zeste Homolog 2 (EZH2) in triple-negative breast cancer.
Anwar chose this project based on intriguing data generated in the Kleer lab for over a decade, which showed that EZH2 promotes breast cancer progression in animal models. They had demonstrated that EZH2 expression in breast cancer tissues signals metastasis.
In normal breast epithelial cells, EZH2 functions in the nucleus as a transcriptional repressor, regulating the genes expressed by normal cells. In this manner, EZH2 controls cell-type identity and differentiation. However, the research team noticed that in triple negative breast cancer, EZH2 has other, previously unconsidered functions. Maria Gonzalez, a Senior Research Specialist and Heather Moore, a previous graduate student in the Kleer lab, had found that EZH2 protein binds to p38 mitogen-activated kinase, which functions by phosphorylating proteins to promote cell survival and migration. Anwar was about to set on his journey to discover the significance of this interaction to breast cancer metastasis.
After nearly two years developing the necessary tools and reagents, Anwar was ready to investigate the consequences of EZH2-p38 binding and EZH2 phosphorylation to the aggressive behavior of triple negative breast cancer. His hard work paid off. The new antibody he generated against phosphorylated EZH2 revealed an exciting new finding: triple negative breast cancer cells showed phosphorylated EZH2 in the cytoplasm, in contrast with the nuclear localization typical of normal cells. He also demonstrated that indeed p38 phosphorylates EZH2 and this event results in EZH2 accumulation in the cytoplasm.
What is the function of phosphorylated EZH2 in the cytoplasm of cancer cells? To address this question, Kleer and Anwar collaborated with several students and their mentors across labs in the tightly-knit MCP. They felt fortunate to have experts on hand and turned to fellow graduate student Jim Ropa, a member of Andrew Muntean Lab, for advice on taking a proteomics approach to test the hypothesis that phosphorylated EZH2 may have binding partners in the cytoplasm, which may promote metastasis of triple negative breast cancer cells. Bioinformatics and proteomics support came from the lab of Alexey Nesvizhskii, PhD. Once several binding partners were discovered, Anwar focused on the interaction between phosphorylated EZH2 and vinculin, a regulator of cell migration and focal adhesion formation. Sierrah Grigsby, a graduate student, and her mentor and MCP program director, Zaneta Nikolovska-Coleska, PhD helped the team elucidate the biochemical and biophysical details of this novel protein-protein interaction.
 Working together, the teams discovered a new mechanism that enables triple-negative breast cancers to move, invade, and metastasize without significant change in the tumor growth, which could lead to the development of new biomarkers to predict metastasis and treatment strategies. One potential therapeutic approach to reduce the metastatic ability of triple negative breast cancer may be to develop small molecules to block the protein-protein interaction between phosphorylated EZH2 and P38, and/or vinculin. The Kleer lab is already working with the Nikolovska-Coleska lab to develop a strategy for creating these molecules and writing grants for funding that will support this work.
Working together, the teams discovered a new mechanism that enables triple-negative breast cancers to move, invade, and metastasize without significant change in the tumor growth, which could lead to the development of new biomarkers to predict metastasis and treatment strategies. One potential therapeutic approach to reduce the metastatic ability of triple negative breast cancer may be to develop small molecules to block the protein-protein interaction between phosphorylated EZH2 and P38, and/or vinculin. The Kleer lab is already working with the Nikolovska-Coleska lab to develop a strategy for creating these molecules and writing grants for funding that will support this work.
“This study has been very special from several perspectives. We made significant progress towards understanding why triple negative breast cancer has an aggressive behavior, trained graduate students, and established a complementary team of close collaborators to move forward”, Kleer says.
Anwar successfully defended his thesis on this work in April and is now finishing his last two years in medical school.
To read the full paper, visit Nature Communications.
 ON THE COVER
ON THE COVER
Breast team reviewing a patient's slide. (From left to right) Ghassan Allo, Fellow; Laura Walters, Clinical Lecturer; Celina Kleer, Professor. See Article 2014Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Autopsy Technician draws blood while working in the Wayne County morgue. See Article 2016Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Sriram Venneti, MD, PhD and Postdoctoral Fellow, Chan Chung, PhD investigate pediatric brain cancer. See Article 2017Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Director of the Neuropathology Fellowship, Dr. Sandra Camelo-Piragua serves on the Patient and Family Advisory Council. 2018Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Residents Ashley Bradt (left) and William Perry work at a multi-headed scope in our new facility. 2019Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Kristine Konopka (right) instructing residents while using a multi-headed microscope. 2020Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Patient specimens poised for COVID-19 PCR testing. 2021Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Pantanowitz demonstrates using machine learning in analyzing slides. 2022Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
(Left to Right) Drs. Angela Wu, Laura Lamps, and Maria Westerhoff. 2023Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Illustration representing the various machines and processing used within our labs. 2024Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Rendering of the D. Dan and Betty Khn Health Care Pavilion. Credit: HOK 2025Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|

MLabs, established in 1985, functions as a portal to provide pathologists, hospitals. and other reference laboratories access to the faculty, staff and laboratories of the University of Michigan Health System’s Department of Pathology. MLabs is a recognized leader for advanced molecular diagnostic testing, helpful consultants and exceptional customer service.