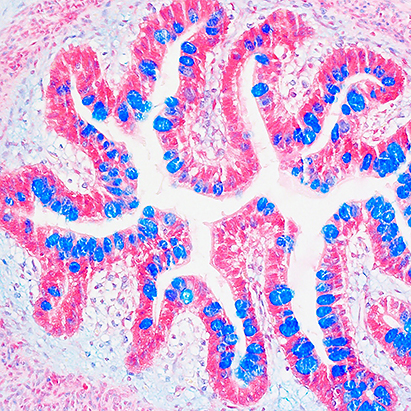
The epithelial lining of the gastrointestinal tract serves as a dynamic and selective barrier permitting uptake of luminal nutrients while restricting pathogen and toxin access to underlying tissue compartments. Epithelial cells provide a unique interface between the luminal environment and immune cells in the sub-epithelial space or lamina propria. Barrier properties are achieved by intercellular junction proteins residing in a series of cell – cell contacts that include tight and adherens junctions as well as desmosomes. These specialized cell-cell contacts consist of over 100 proteins that actively participate in the complex process of controlling barrier function and epithelial homeostasis. We are interested in identifying and functionally characterizing these protein constituents of intercellular junctions.
Many acute and chronic inflammatory conditions result in compromised epithelial barrier function with enhanced permeability and increased exposure of mucosal immune cells to microbial antigens. Under these conditions, there is release of cytokines and chemokines that act to further perturb epithelial barrier function. Breakdown of the epithelial barrier has been shown to play a key role in the pathogenesis of several chronic inflammatory disorders of the intestine collectively referred to as Inflammatory Bowel Disease (IBD). Our studies are aimed at identifying molecular mechanisms by which such immune mediators control intercellular junction protein dynamics and epithelial barrier function. The ultimate objective is to develop strategies to strengthen the epithelial barrier and restore mucosal homeostasis under pathologic conditions.
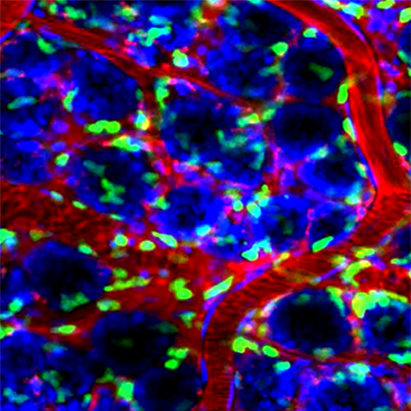
A major component of many inflammatory diseases of the gastrointestinal, respiratory, and urinary tract is migration of large numbers of leukocytes termed neutrophils (PMN) across the epithelium. While a necessary component of the inflammatory response against invading pathogens, pathologic PMN transmigration also commonly occurs in response to unknown stimuli in a number of diseases such as IBD. Under these conditions, disease symptoms are directly related to leukocyte effects on the epithelial barrier and epithelial cell function. Conversely, it is now appreciated that epithelial mucosal homeostasis is highly dependent upon relationships between the epithelial barrier and leukocytes, regulated, in part, by cell- cell contact and release of factors that initiate subsequent signaling events. While much has been learned about mechanisms of leukocyte migration from the circulation, relatively little is known about the receptors that regulate leukocyte-epithelial interactions. We are interested in understanding the structure and function of leukocyte (PMN) and epithelial receptors that mediate leukocyte transepithelial migration, and understanding the molecular basis and functional consequences of leukocyte-epithelial crosstalk. Importantly, identification and characterization of proteins that mediate leukocyte-epithelial interactions will provide new strategies for mucosal targeted therapeutics with a potential benefit of less systemic side effects than conventional therapies for conditions such as Inflammatory Bowel Disease.
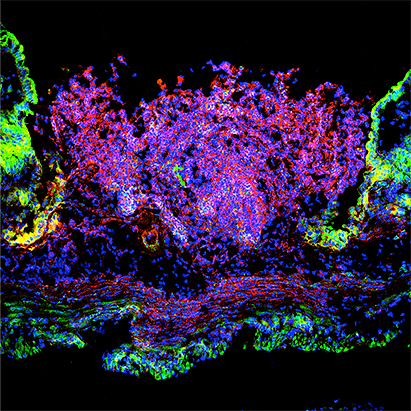
Mucosal wounds and barrier disruption are characteristic of multiple clinical conditions that include inflammatory diseases (secondary to infection or idiopathic as in IBD), compromised circulation, irradiation and following surgical procedures. Epithelial wound closure involves concerted integration of epithelial cell proliferation and migration and requires a complex network of epithelial receptors (G-protein coupled receptors such as Formyl peptide receptors) and signaling events derived from pro-resolving mediators released by epithelial cells, leukocytes and luminal microbiota. We are interested in elucidating basic molecular mechanisms that control mucosal wound repair and in developing novel therapeutic strategies aimed at promoting repair of the damaged epithelium.
 ON THE COVER
ON THE COVER
Breast team reviewing a patient's slide. (From left to right) Ghassan Allo, Fellow; Laura Walters, Clinical Lecturer; Celina Kleer, Professor. See Article 2014Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Autopsy Technician draws blood while working in the Wayne County morgue. See Article 2016Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Sriram Venneti, MD, PhD and Postdoctoral Fellow, Chan Chung, PhD investigate pediatric brain cancer. See Article 2017Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Director of the Neuropathology Fellowship, Dr. Sandra Camelo-Piragua serves on the Patient and Family Advisory Council. 2018Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Residents Ashley Bradt (left) and William Perry work at a multi-headed scope in our new facility. 2019Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Kristine Konopka (right) instructing residents while using a multi-headed microscope. 2020Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Patient specimens poised for COVID-19 PCR testing. 2021Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Pantanowitz demonstrates using machine learning in analyzing slides. 2022Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
(Left to Right) Drs. Angela Wu, Laura Lamps, and Maria Westerhoff. 2023Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Illustration representing the various machines and processing used within our labs. 2024Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Rendering of the D. Dan and Betty Khn Health Care Pavilion. Credit: HOK 2025Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|

MLabs, established in 1985, functions as a portal to provide pathologists, hospitals. and other reference laboratories access to the faculty, staff and laboratories of the University of Michigan Health System’s Department of Pathology. MLabs is a recognized leader for advanced molecular diagnostic testing, helpful consultants and exceptional customer service.