Study Regarding Inhibition of Class I PI3K Published in Journal of Cell Biology
By Camren Clouthier | October 30 2020 Research by the Department of Pathology's Dr. Richard Miller and Dr. Joseph Endicott was just published in the Journal of Cell Biology. The study focuses on the inhibition of class I PI3K and how it enhances chaperone-mediated autophagy (CMA).
Research by the Department of Pathology's Dr. Richard Miller and Dr. Joseph Endicott was just published in the Journal of Cell Biology. The study focuses on the inhibition of class I PI3K and how it enhances chaperone-mediated autophagy (CMA).
Experts first discovered that chaperone-mediated autophagy is the most selective form of lysosomal proteolysis, where individual peptides, recognized by a consensus motif, are translocated directly across the lysosomal membrane. Additionally, CMA regulates the abundance of many disease-related proteins, with causative roles in neoplasia, neurodegeneration, hepatosteatosis, and other pathologies relevant to human health and aging.
"At the lysosomal membrane, CMA is inhibited by AKT-dependent phosphorylation of the CMA regulator GFAP," describes Dr. Miller. "The INS-PI3K-PDPK1 pathway regulates Akt, but its role in CMA is unclear. Here, we report that inhibition of class I PI3K or PDPK1 activates CMA."
To test whether inhibition of intracellular signaling downstream of INS is sufficient to activate CMA, the research team hand-selected three structurally distinct inhibitors of all four isoforms and p110-δ, i.e., buparlisib, pictilisib, and copanlisib. PI3K inhibitors commonly have off-target effects, varying with the inhibitor’s structure. Researchers theorized that if multiple chemically distinct inhibitors consistently give the same biological effect, then it is unlikely that the measured result arises from inhibition of off-target kinases. The first experiment was performing dose curves in NIH3T3 cells to identify the lowest dose of each inhibitor that maximizes AKT inhibition (to reduce the chances of nonspecific kinase inhibition), as judged by the phosphorylation status of AKT and its own target, GSK3β. Subsequent experiments in NIH3T3 cells were performed using 2 µM buparlisib, 500 nM pictilisib, and 50 nM copanlisib, unless otherwise specified.
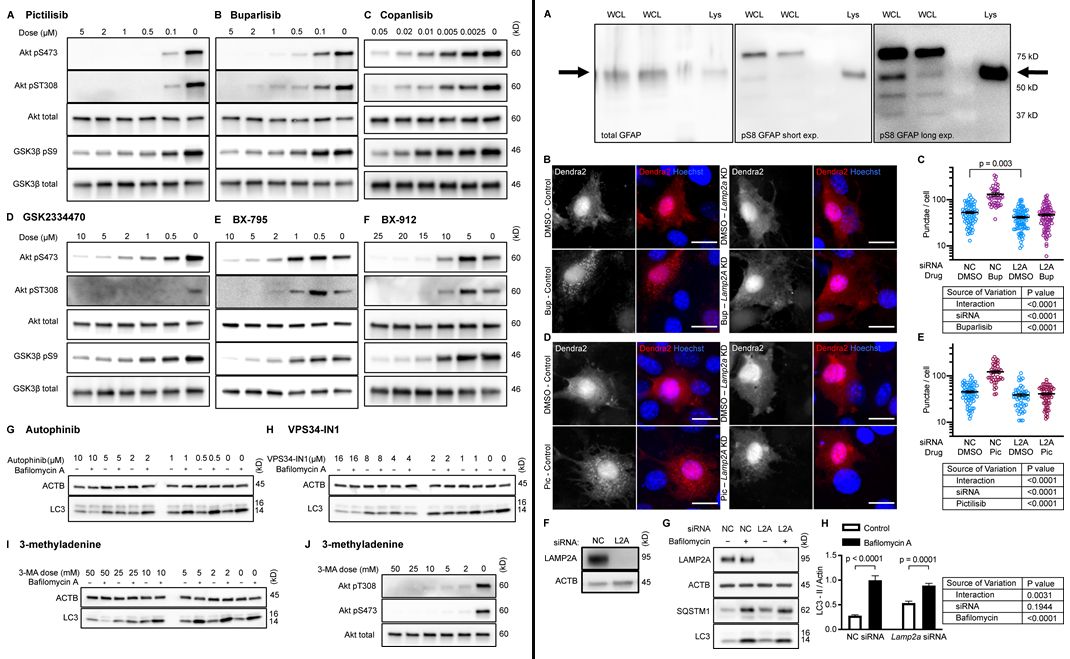 Next, the team tested whether inhibiting p110 is sufficient to decrease phosphorylation of GFAP. Initially, it was found that GFAP and GFAP phospho-S8 (pGFAP) antibodies can detect multiple bands in whole tissue lysates and whole cell lysates, but that the antibodies detect a single band on lysosomes. The pGFAP band in whole cell lysate that corresponds in size to the lysosomal band correlates very tightly with lysosomal pGFAP and CMA activity. Researchers identified the lysosomal pGFAP and GFAP bands in NIH3T3 cells as the ∼55 kD bands.
Next, the team tested whether inhibiting p110 is sufficient to decrease phosphorylation of GFAP. Initially, it was found that GFAP and GFAP phospho-S8 (pGFAP) antibodies can detect multiple bands in whole tissue lysates and whole cell lysates, but that the antibodies detect a single band on lysosomes. The pGFAP band in whole cell lysate that corresponds in size to the lysosomal band correlates very tightly with lysosomal pGFAP and CMA activity. Researchers identified the lysosomal pGFAP and GFAP bands in NIH3T3 cells as the ∼55 kD bands.
All three p110 inhibitors sharply reduced phosphorylation of AKT on T308 and S473 and MAPKAP1 on T86, as expected. GFAP phosphorylation was also significantly reduced by all three p110 inhibitors, relevant to their solvent controls. A reduction in GFAP phosphorylation suggests the hypothesis that CMA activity is increased.
To test for CMA activation in response to buparlisib, pictilisib, and copanlisib, the team of researchers used a genetically encoded CMA reporter, consisting of the N terminus of RNase A fused to the photo-switchable fluorescent protein Dendra2. Results found that all three p110 inhibitors caused a very clear increase in the number of cytosolic Dendra2 puncta, suggesting an increase in CMA.
Finally, to verify that the increase in puncta is not attributable to another translocation event, the experiment was repeated in cells treated with siRNA targeting exon 8A of the Lamp2 transcript (exon 8A is unique to the Lamp2a transcript, and not found in Lamp2b or Lamp2c) or a nontargeting siRNA control. Tested were buparlisib and pictilisib in the siRNA-treated cells, evidencing that the increase in the number of Dendra2 puncta in response to both drugs was completely blocked in cells with Lamp2a knocked down. A representative Western blot controlling for successful LAMP2A protein depletion.
Ultimately, the results suggested that selective inhibition of class III PI3Ks does not activate CMA. Isolated liver lysosomes from mice treated with either of two orally bioavailable class I PI3K inhibitors, pictilisib or buparlisib, display elevated CMA activity, and decreased phosphorylation of lysosomal GFAP, with no change in macroautophagy. Together, this data suggest that inhibiting p110 causes an increase in CMA, whether or not macroautophagy is also inhibited.
Overall, this study represents an important step towards developing therapeutics that may fight many forms of cancer. "The findings [of this study] represent an important first step in repurposing class I PI3K inhibitors to modulate CMA in vivo," notes Miller.
--
The full publication in the Journal of Cell Biology is accessible here.
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
