
Senior Technologists: |
|
|
Ellen Bassett, Media Receiving Telephone: (734) 936-6827 |
|
|
|
Specimen Processing receives specimens from a variety of hospitals, clinics, and clients. Once received, this area evaluates specimens for proper test order, transport media, and collection methodology. Specimens are then setup on media corresponding to test order(s) and incubated accordingly. Specimen Processing also reads Gram stains of direct smears from specimens. This area of the lab is staffed 24 hours a day, 365 days a year.
Adjacent to Specimen Processing is Media Receiving. Media Receiving orders and receives supplies for all the Clinical Microbiology Laboratories. This area also performs quality control on reagents, media, and bacterial identification kits as they are received. Media Receiving works with laboratory leadership and hospital purchasing to obtain contracts with companies that provide quality products at reasonable prices.
Lead Technologists |
|
| Senior Technologists: |
Connie Brenke, Susceptibility Testing Telephone: (734) 936-66831
Kathleen Cullen, Aerobes and Special Bacteriology Telephone: (734) 936-7284 |
|
Medical Technologists 2: |
Lily Keenan, Anaerobes Telephone: (734) 232-5598
Becky Ott, Blood Cultures Telephone: (734) 936-7284 |
The bacteriology area performs identification and susceptibility tests on a variety of micro-organisms isolated from patient specimens. Bacterial cultures are read on day and afternoon shifts to ensure rapid reporting to the clinicians.
MALDI-TOF (Matrix Assisted Laser Deionization-Time of Flight) is a mass spectrometry instrument used in the laboratory for identification of microbial organisms. MALDI-TOF has reduced the turnaround time for identification of organisms compared to conventional methods. This system may reduce identification time from hours to minutes for many isolates.
Patient specimens are divided by type to provide a focused attention to the potential pathogens and flora types expected from each collection site, including blood and spinal fluids, body fluids and wounds, urogenital cultures, respiratory cultures and urine cultures.
Blood cultures are incubated on an automated instrument. These cultures are monitored continuously for at least 5 days. Reports are issued automatically after 24 hours of incubation for all negative blood cultures. Automatic final negative results are issued after 5.5 days. There are no intervening updates on negative cultures.
Susceptibility testing is performed using broth microdilution or disc diffusion. The susceptibility testing area works in close association with the pharmacy department to provide appropriate antibiotic results for optimal patient care.
Senior Technologist |
Stephanie Hillman Telephone: (734) 936-6829 |
Mycology offers to test for speciation of fungal organisms and for performing yeast in vitro susceptibility testing.
Mycobacteriology offers Mycobacteria species identification and select organism susceptibility testing.
Medical Technologist 2 |
Saira Ramirez Telephone: (734) 232-5601 |
Parasitology performs routine and STAT examination on a wide range of specimens. STAT examination of blood films is available 24 hours a day, 365 days a year. Our lab routinely performs tests including ova and parasite examination of stool, pinworm testing, stool antigen testing for Giardia, rapid antigen testing of urine for Trichomonas, and identification of medically important ticks and worms. Testing can also be performed on specimens from other body sites like sputum, urine, duodenal aspirates, etc. Our lab works in close association with health care providers to optimize collection and identification of parasites.
Lead Technologist |
Lesley Lebioda Telephone: (734) 936-6842 |
Molecular Diagnostics is a rapidly evolving area of the Clinical Microbiology Laboratory. It routinely performs a high volume of testing for human papillomavirus, Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas, Mycoplasma and Clostridium difficile toxin. Molecular quantitative testing is performed for cytomegalovirus, HIV, hepatitis C, and hepatitis B. Qualitative tests include the detection of Bordetella pertussis and B. parapertussis, Pneumocystis jiroveci, Legionella, herpes simplex virus I and II, hepatitis C genotyping, respiratory viruses including influenza A, influenza B, respiratory syncytial virus, parainfluenza 1-3, and human metapneumovirus and gastrointestinal viruses, parasites and bacteria including norovirus, Salmonella and Giardia.
 ON THE COVER
ON THE COVER
Breast team reviewing a patient's slide. (From left to right) Ghassan Allo, Fellow; Laura Walters, Clinical Lecturer; Celina Kleer, Professor. See Article 2014Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Autopsy Technician draws blood while working in the Wayne County morgue. See Article 2016Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Sriram Venneti, MD, PhD and Postdoctoral Fellow, Chan Chung, PhD investigate pediatric brain cancer. See Article 2017Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Director of the Neuropathology Fellowship, Dr. Sandra Camelo-Piragua serves on the Patient and Family Advisory Council. 2018Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
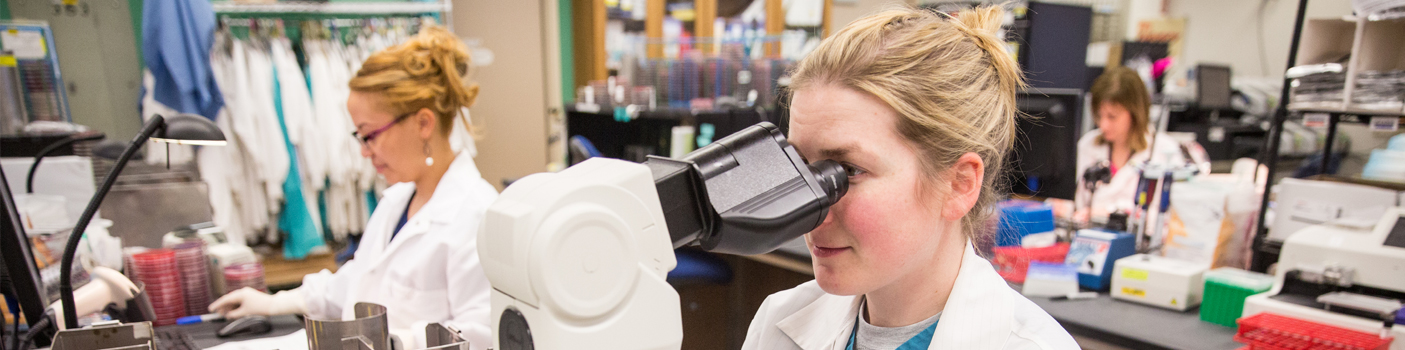
Residents Ashley Bradt (left) and William Perry work at a multi-headed scope in our new facility. 2019Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Kristine Konopka (right) instructing residents while using a multi-headed microscope. 2020Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Patient specimens poised for COVID-19 PCR testing. 2021Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Pantanowitz demonstrates using machine learning in analyzing slides. 2022Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
(Left to Right) Drs. Angela Wu, Laura Lamps, and Maria Westerhoff. 2023Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Illustration representing the various machines and processing used within our labs. 2024Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Rendering of the D. Dan and Betty Khn Health Care Pavilion. Credit: HOK 2025Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|

MLabs, established in 1985, functions as a portal to provide pathologists, hospitals. and other reference laboratories access to the faculty, staff and laboratories of the University of Michigan Health System’s Department of Pathology. MLabs is a recognized leader for advanced molecular diagnostic testing, helpful consultants and exceptional customer service.