Study provides mechanistic link of diet to inhibition of colitis in Crohn's disease
By Lynn McCain | November 16 2023 Crohn’s disease (CD), one of the major forms of Inflammatory Bowel Disease, is a chronic relapsing intestinal disorder that affects millions of people globally. The cause of CD is unknown, but it is thought to result from a dysregulated immune response against environmental factors, including intestinal microbes, in genetically susceptible hosts.
Crohn’s disease (CD), one of the major forms of Inflammatory Bowel Disease, is a chronic relapsing intestinal disorder that affects millions of people globally. The cause of CD is unknown, but it is thought to result from a dysregulated immune response against environmental factors, including intestinal microbes, in genetically susceptible hosts.
The critical role that the intestinal microorganisms, collectively known as the gut microbiota, plays in the pathogenesis of CD has been investigated over the past two decades. Given the importance of the gut microbiota in triggering CD, the development of therapeutic approaches that target disease-causing intestinal microbes may provide a unique approach to the treatment of this disease.
Diet is one of the available tools that can be used to modify the gut microbiota. As of now, there are no clear evidence-based dietary recommendations for the management of CD, except for exclusive enteral nutrition (EEN). EEN is a complete liquid formula diet that contains all nutritional requirements but is typically free of or low in plant fiber. EEN is a first line therapy for mild to moderately active pediatric CD, which induces remission in up to 85% of cases. However, the mechanism by which EEN induces clinical remission in children with CD remains unclear.
In a new manuscript published in the journal Cell Host & Microbe, the senior author Dr. Roberta Caruso, MD, PhD, Research Assistant Professor in the Department of Pathology, used a spontaneous model of microbiota-dependent CD-like colitis to study the mechanism by which fiber-free diet regulates intestinal inflammation. Caruso was leading the project in the laboratory of Dr. Gabriel Nuñez.
The team used a novel mouse model of colitis, which is based on genes associated with CD and recapitulates multiple hallmarks of the human disease. Intestinal inflammation in this model is induced by a single disease-causing microbe, Mucispirillum schaedleri, a mucus-dwelling anaerobe found both in mice and humans.
They found that the administration of a fiber-free diet prevents the development of colitis and inhibits intestinal inflammation in colitic animals. The results were somewhat unexpected, given that the administration of low fiber diet was associated with thinning of the mucus layer and increased mucus permeability in this model of colitis. Remarkably, the team identified a distinct and unique mechanism through which the absence of dietary fiber inhibits intestinal inflammation by targeting the localization and metabolism of the pathobiont M. schaedleri. Metatranscriptomic analysis of mice colonized by a complex microbiota maintained on a fiber-deficient diet showed that the absence of dietary fiber reduces the availability of nutrients and impairs the dissimilatory nitrate reduction to ammonia (DNRA) metabolic pathway of M. schaedleri, leading to its exclusion from the mucus layer and disease remission.
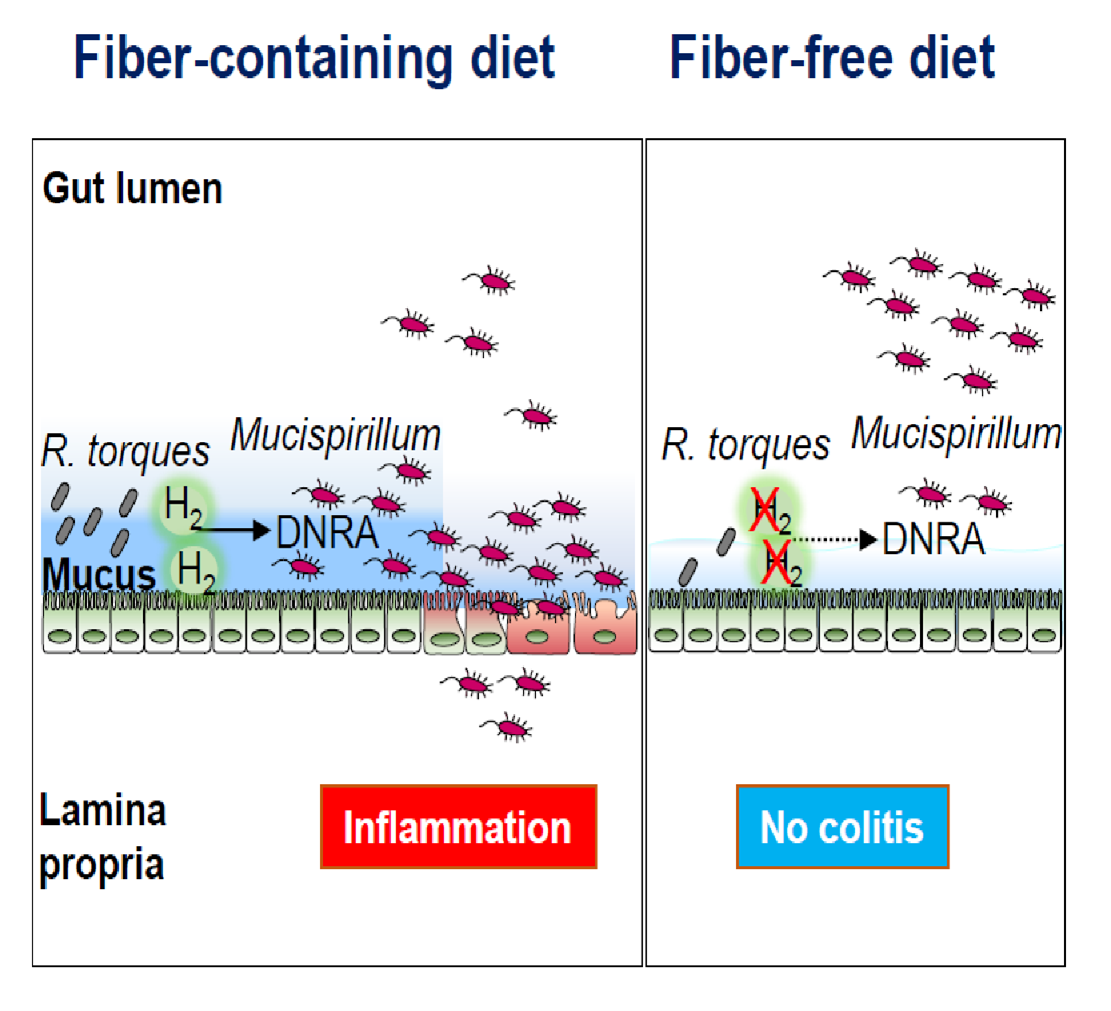 Although M. schaedleri is a mucus-dwelling bacterium, it lacks the enzymatic machinery necessary for the utilization of host-derived polysaccharides, suggesting that this bacterium may utilize breakdown products produced by mucus-degrading microbes. To test this hypothesis, the team first examined the bacterial species that were affected by the exclusion of dietary fiber and found that the abundance of several members of the Lachnospiraceae family, including Ruminococcus torques, was reduced under fiber-free conditions. Next, in collaboration with Dr. Eric C. Martens, Professor in the Department of Microbiology and Immunology, the group found that the mucin-degrading R. torques regulates the growth of Mucispirillum in vitro and in vivo.
Although M. schaedleri is a mucus-dwelling bacterium, it lacks the enzymatic machinery necessary for the utilization of host-derived polysaccharides, suggesting that this bacterium may utilize breakdown products produced by mucus-degrading microbes. To test this hypothesis, the team first examined the bacterial species that were affected by the exclusion of dietary fiber and found that the abundance of several members of the Lachnospiraceae family, including Ruminococcus torques, was reduced under fiber-free conditions. Next, in collaboration with Dr. Eric C. Martens, Professor in the Department of Microbiology and Immunology, the group found that the mucin-degrading R. torques regulates the growth of Mucispirillum in vitro and in vivo.
Further, in collaboration with Dr. Thomas M. Schmidt, Professor in the Department of Microbiology and Immunology, Caruso showed that the mucolytic R. torques produced H2, a fermentation byproduct, which, in turn, supported Mucispirillum growth via the DNRA pathway. The group also found that fiber exclusion reduced the expression of hydrogenases in the mucus layer and altered the levels of hydrogenase-expressing Lachnospiraceae, which, in turn, limited the availability of nutrients necessary for Mucispirillum colonization in the mucus layer.
This study introduces a novel concept that diet can control the proximity of pathobionts to the host epithelium through mucolytic microbes that have the ability to regulate the growth and metabolism of pathobionts in the mucus layer.
********
Citation: Kuffa et al., Fiber-deficient diet inhibits colitis through the regulation of the niche and metabolism of a gut pathobiont, Cell Host & Microbe (2023), https://doi.org/10.1016/j.chom.2023.10.016
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
